FREQUENTLY ASKED QUESTIONS
GRX-917 is a proprietary (patent-protected until 2036), improved version of Etifoxine, an anxiety drug first approved in France 39 years ago. GRX-917 is a deuterated version of Etifoxine (specific hydrogen atoms were replaced by isotopic deuterium (heavy hydrogen) atoms). Like the vast majority of deuterated drug analogs, GRX-917 retains the same mechanism of action and pharmacological properties as etifoxine, because their biological properties are identical.
Clinically, GRX-917’s speed of onset, efficacy and side effects profile will be identical to the original drug. However, the pharmacokinetic profile is significantly improved as a result of reduced metabolism due to deuterium stabilization. Critically, GRX-917’s metabolism remains identical to etifoxine, but with a reduced rate. This is expected to lead to lower and/or less frequent dosing and more consistent exposure, leading to better efficacy and less potential side effects/adverse effects than the original drug.
GRX-917’s main mechanism of action increases the amount of naturally occurring neurosteroids (like allopregnanolone) by activation of TSPO on mitochondria. This increase of endogenous neurosteroids occurs in areas in the brain where they are used to activate GABA receptors, slowing dysregulated brain activity in diseases like anxiety and depression, but minimizing the typical side effects of GABA drugs, like benzodiazepines. Additionally, increases of neurosteroids acting at other receptors have been found to minimize neuroinflammation, affect neuroregeneration and modulate the activation of microglia – potentially key elements in therapeutics for Alzheimer’s Disease, Multiple Sclerosis and Parkinson’s Disease.
Etifoxine (and therefore GRX-917) has been shown through multiple published, peer reviewed clinical studies, to have comparable rapid onset and efficacy to leading benzodiazepines, including like alprazolam (Xanax™) and lorazepam (Ativan™).
Over 40 years of research and clinical data on etifoxine (and therefore GRX-917) has shown that the drug has little to no side effects:
- No Sedation (some people say slight sedation on day one which quickly remits)
- No Cognitive Impairment
- No Ataxia
Etifoxine (and therefore GRX-917) has an excellent safety/pharmacological profile, based on a safety profile review by ANSM in France (2000-2012) which analyzed over 14 million prescriptions of etifoxine.
- Non addictive (no cases of abuse, misuse or pharmacodependence)
- No tolerance, withdrawal or rebound
- Low ADRs (21 per million) based on +14 million prescriptions analyzed in France (2000-2012)
- SAEs are very rare and occur less frequently than with both first line and second-line anxiolytics
Early Beginnings
The original, non-deuterated drug (etifoxine) was originally developed to compete with tranquilizing and sedative BZs of the 1960’s, such as Valium and Librium. But it was mischaracterized as a mild benzodiazepine, because of its lack of typical benzodiazepine side effects, like sedation and ataxia. Hoechst sold the rights to the drug, without knowing its true efficacy and unaware of its mechanism of action. A small pharmaceutical distributor has been producing and distributing the drug off-patent since 1979, in limited markets. The drug was never developed in the U.S. or broader European markets.
Discovering the Extraordinary Mechanism of Action
Contrary to what was originally believed, French researchers in the 2000’s discovered that etifoxine did not interact at the benzodiazepine site on GABA receptors! It took sophisticated modern research techniques to reveal a truly significant discovery: The drug increases the levels of natural neurosteroids, like allopregnanolone (ALLO), a highly potent and effective modulator of GABA channels, and that the clinically observed effects could be reversed in pre-clinical experiments by blocking the natural synthesis of these steroids. Scientists quickly took notice of this unique biological action and began to explore etifoxine in models of other indications where neurosteroids (like ALLO) had shown efficacy: Alzheimer’s Disease, Multiple Sclerosis, Parkinson’s Disease and traumatic brain injury, to name a few.
Etifoxine – No Patent Protection – Not Viable
The stage was set for an old drug to be rejuvenated, but the original patents had expired and most of the therapeutic indications had already been disclosed (or patented). Without patent protection, there was no business rationale to re-develop the drug in any new markets, which explains why etifoxine was never introduced into the United States. This is the rationale behind the discovery and development of GRX-917: a vastly improved variant of etifoxine, with patent protection.
The first-line anxiety medications (SSRIs/SNRIs) take 4-6 weeks to take effect and work in less than 50% of anxiety patients.
The second line anxiety medications (benzodiazepines) are highly addictive, have serious side effects and historically have been involved in approximately 1/3 of fatal prescription overdoses in the United States.
Neurosteroids, like allopregnanolone (ALLO), are natural signaling molecules made in the brain from cholesterol and are essential for normal brain biochemistry. They can induce potent anxiolytic, antidepressant, anticonvulsant, and analgesic effects, mainly through interaction with the GABA channel, the main inhibitory system in the brain.
In addition to these psychopharmacological effects, neurosteroids also demonstrate neuroprotective, neurotrophic, and anti-inflammatory effects in several animal models of neurodegenerative diseases, mainly by shutting down overactive immune cells (like microglia). Chronic activation of the brain’s resident immune cells results in neuroinflammation, a common feature of many neurodegenerative diseases which is implicated in their onset and progression.
Altered levels of neurosteroids such as ALLO have been measured in many neuropsychiatric and neurological disorders (anxiety, depression, post-partum, post-traumatic stress disorder, schizophrenia, alcohol dependence, epilepsy, Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, Niemann-Pick type C disease, diabetic neuropathy, traumatic brain injury, stroke and retinal degenerative disorders) both in humans and animal models.
Both preclinical and clinical studies demonstrate a therapeutic potential for neuroactive steroids to both alleviate symptomology, and/or modify the disease state in a variety of CNS disorders.
The human brain is a complex network of billions of interconnected cells, organized in many networks communicating with one another. Some CNS disorders arise from dysregulation in the modulation of these networks, either by altered levels of signaling molecules like neurosteroids or the receptors they target.
Boosting the brain’s natural production of neurosteroids to restore normal neurosteroid tone represents a better and potentially more natural therapeutic approach for the treatment of these complex CNS disorders.
Drugs that naturally enhance the brain’s concentration of neurosteroids produce a full spectrum of pharmacological actions, unlike unnatural synthetic versions, which only target specific receptors. Key to natural enhancement of neurosteroids is the preservation of the spatial and temporal nature of neurotransmission. This results in less side effects, because the brain controls, with exquisite precision, what, where and when neurosteroids are being produced, unlike drugs that are given exogenously.
The Company has used deuteration technology to create a new and improved version of etifoxine, deuterated etifoxine (GRX-917), that has composition of matter patent protection, until 2036.
On August 29, 2019 GABA Therapeutics Inc. secured up to $15.5 million of Series A investment from Atai Life Science AG, which will take the GRX-917 program through Phase 2a.
ATAI Life Sciences AG is a global biotech platform and company builder, based in Berlin, London and New York, focused on curing mental health disorders. Clearly there are synergies between GABA Therapeutics and Atai Life Sciences, and both companies aim to collaborate and share resources where commercially viable.
GABA Therapeutics will be conducting the following Phase 1 studies:
- Initially etifoxine will be studied to measure its ability to increase endogenous neurosteroids in man.
- Secondly, GRX-917 (deuterated etifoxine) will be studied for toxicology, safety, dosing and its ability to increase endogenous neurosteroids in man.
GABA Therapeutics is conducting Phase 1 studies in Australia for the following reasons:
- Australian law allows clinical studies to commence before an IND is submitted, substantially expediting the program, and
- Current Australian tax law provides for a 43.5% tax rebate on eligible clinical related costs.
Neurosteroids could benefit the following neuropsychiatric and neurologic disorders:
- Green (1-11) – preclinical studies using Etifoxine which have demonstrated efficacy
- Blue (12) – Etifoxine is prescribed for depression in France (off-label), and clinical data demonstrates ALLO efficacy
- Purple (13-20) – preclinical and clinical studies suggest increasing biosynthesis of neurosteroids, like ALLO, could benefit these conditions
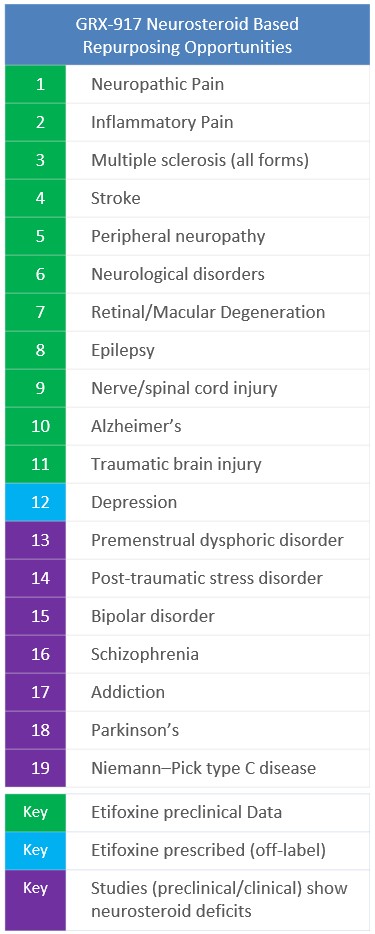
Management has not yet determined what indication will be pursued in the proof-of-concept study. However, etifoxine is prescribed (off-label) in Europe and other countries for depression. Furthermore, recent clinical studies have shown that increased neurosteroids have therapeutic effect for various depression indications, including Major Depressive Disorder and Post-Partum Depression. Accordingly, both indications are being evaluated as potential candidates for this study.
Anxiety, which is frequently comorbid with depression, is the largest mental health issue in North America. It is estimated that one third of the North American adult population experiences anxiety issues, yet only one third of sufferers receive treatment, of whom only 10% experience any form of remission. This is largely due to limitations in current therapies. Anxiety affects 48 million patients in the United States. The US market for anxiety prescriptions was $8.4 billion last year. The global market for anxiety medications was $13.7 billion last year. In 2018, there were approximately 140 million prescriptions of anxiety medications in the United States. (1)
(1) IQVIA market data – 2018
Management intends to IPO once it completes Phase 1 and its proof-of-concept study to demonstrate efficacy. Data gathered measuring GRX-917’s ability to increase neurosteroids, along with efficacy data, should provide for a highly anticipated public offering.
- “In primary care settings benzodiazepines are frequently prescribed for psychiatric symptoms, despite their adverse event profile and their potential risk for dependence. The non-benzodiazepine drug etifoxine is a promising agent insofar as it is not associated with dependence. Its efficacy and safety have been evaluated in three double-blind randomized clinical trials in comparison with non-benzodiazepine (buspirone) and benzodiazepines (lorazepam and alprazolam). The three trials point to the anxiolytic properties of etifoxine, demonstrating an overall clinical improvement of patients, and no risk for dependence or rebound effect.”
–D. Stein (European Psychiatry 2015)
- “In our study, compared to lorazepam (Ativan) group, more patients were responders to treatment in etifoxine group, and more patients were markedly improved (scores 1 and 2 in CGI-global improvement). Our study was designed to compare by a non-inferiority test two psychotropic drugs on the bases of HAM-A differences and led to the conclusion of non- inferiority of etifoxine compared to lorazepam. Clinical response rates, classically used in trials on anxiolytic drugs, were found in favor of the etifoxine group. Another point to underline is the rapid onset of therapeutic effect in etifoxine recipients, from the first week of treatment, as in lorazepam group. This is important to consider.”
–O. Blin (Hum Psychopharmacol Clin Exp 2006)
- “The safety profile of etifoxine and alprazolam (Xanax) is again consistent with prior work on these agents. Thus, it is notable that treatment-emergent adverse events were more likely to occur in the alprazolam group, and these were significantly more likely to be considered treatment-related by clinicians. Furthermore, more CNS-related adverse events, such as somnolence or sedation, were reported in the alprazolam group, although this difference did not reach statistical significance. Finally, the higher proportion of patients with adverse events in the alprazolam group was particularly apparent after treatment discontinuation. These findings are consistent with consensus statements that highlight cognitive adverse events and the potential for dependence with benzodiazepines. In contrast, etifoxine has a relatively safe adverse event profile, and has the significant clinical advantage of having anxiolytic properties, while not being associated with potential for dependence.”
–D. Stein (Adv Ther 2015)
- The treatments available so far are very effective but, in many cases, it is not possible to treat their symptoms without suffering adverse effects. In that sense, Professor Dr. Márquez, Director of ADINEU (Assistance, Teaching and Research in Neuroscience) and Honorary President of the AATA (Argentine Association of Anxiety Disorders) said: “Since last year we started to explore in Argentina the efficacy of a new anxiolytic that has a long history in several European countries but that had not been available to us until now. What is most striking about this drug is the data about its tolerability, which is the current challenge of the treatment of anxiety.”
- “It is a new alternative because although psychiatrists have enough tools to ensure control of the anxious manifestations of almost all patients, in many cases it was necessary to pay a high cost in adverse effects on quality of life such as sedation, induction of sleep, excessive muscle relaxation, weight gain, sexual dysfunctions and sometimes even phenomena of habituation and effects on memory and other cognitive processes.”
-Dr. Miguel Márquez Psychiatrist and Director of ADINEU, Center dedicated to Assistance, Teaching and Research in Neuroscience, University of Buenos Aries (Google Translated)
- “Study of effectiveness and adverse effects of etifoxine compared with clonazepam (Klonopin) in the treatment of anxiety disorders in primary care patients: We contacted these patients in the waiting room and, in order to obtain the sample, we treated 3,800 patients, who were screened and the diagnosis of anxious disorder was later confirmed with CIDI. Then 294 patients were invited to participate, who were divided into two groups: one experimental and one control. In the background it is a randomized, double-blind, controlled trial with parallel groups. Once the person entered the study, they were treated for 12 weeks, then the therapy was discontinued and after 24 weeks, that is, 12 weeks later, the test batteries were re-applied. All of the above for the purpose of determining if the medication produced an effect and, eventually, how long that effect was maintained once the treatment was finished. It is a clinical trial with the characteristics of being a double-blind, randomized study. The results seem clear to us to the extent that etifoxine appears as a drug that is more effective and produces fewer side effects than clonazepam, which we chose as the object of study because it is the most widely used medicine in Chile as anxiolytic at all levels health So, it seemed relevant to compare a relatively new and new molecule, such as etifoxine, with the drug that is widely used. Etifoxine is more effective and produces fewer side effects, and the results are maintained in favor of etifoxine at 24 weeks.”
– Dr. Benjamin Vicente, LXXI Chilean Congress of the Society of Neurology, Psychiatry and Neurosurgery 2016 (Sonepsyn) (Google Translated)
